

Our Capabilities
With over 25 years of experience our in-house multi-disciplinary team specialises in identifying and implementing opportunities for improvement in molecular diagnostics through the creation of bespoke and novel technological solutions to improve global health outcomes.
We have capabilities in all key areas to suit a broad scope of applications and requirements; even the most complex and challenging of ones.

Mechanical Engineering
Our mechanical engineering team specialises in the early stages of rapid prototyping and test rigs to demonstrate proof of concept all the way through to design for manufacture and full production systems.
They have extensive experience in developing specialist components and integrating complex sub-assemblies to form complete functional systems. Areas of expertise include CAD modelling, injection moulding, technical drawings, thermal control & cycling, design for manufacture and system integration. Our mechanical workshop is complete with CNC machining and 3D printing facilities.
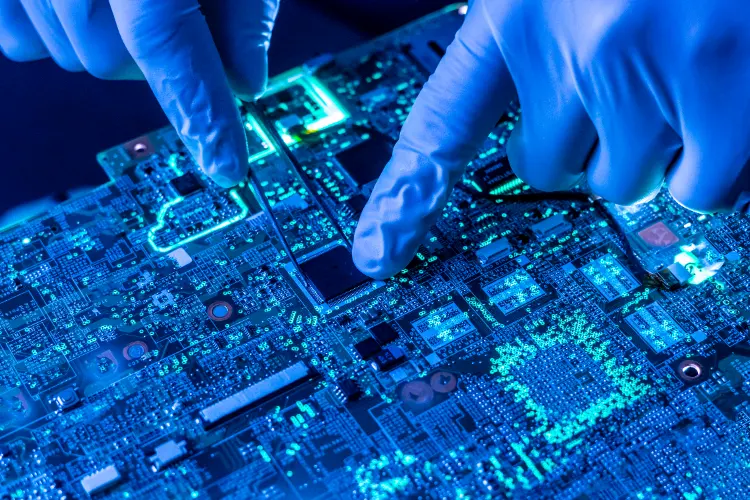
Electronic Engineering
Our electronic engineering team provides full life-cycle development and integration of electronical components.
This includes component specification, testing & troubleshooting, PCB design & production, system integration, inter and intra system communications, PID/AI control and micro-controller firmware.

Software Development
We develop intuitive, intelligent and user friendly software for molecular diagnostic systems in accordance with regulatory standards including IEC 62304.
Our software is designed to simplify the results calling process and empower users with minimal training to utilize pioneering molecular technologies to effectively triage patients when working with clinicians.
We also provide software packages for expert users who require full access to testing parameters and data for detailed in-depth analysis.
Optical Detection Systems
We have extensive experience in developing optical detection systems used for RT-qPCR applications that are sensitive, robust, low cost and suited to in-field use. This includes both laser and LED based optical detection systems.
We can specify the required components for optical detection systems such as light sources, spectrometers, filters, optical fibres, light guides and mounting solutions. With dark room facilities onsite optical detection systems can be tested and validated in accordance with specified performance requirements.


Assay Design & Optimisation
We have particular expertise in performing RT-qPCR direct from crude sample types including whole blood, saliva and swabs. These tests are designed to be used in-field in decentralised settings whilst the patient waits (At Patient Testing) and are capable of high sensitivity multiplex detection of panels of infectious diseases. Our team of molecular biologists are able to provide an assay development and validation pipeline approach.
Typical turnaround time is 6 weeks from receipt of sequencing to validation in accordance with our certified ISO 13485 quality management system. This allows new or existing assays to be rapidly developed, optimised, validated and deployed at scale (particularly important in responding to emerging High Consequence Infectious Diseases). Assays can be configured and optimised for specific sample mediums and facilitate multi-plex panels (e.g. respiratory and blood borne) in accordance with defined user requirements.
Manufacturing
Design for Manufacture and Assembly (DfMA) is a driving principle and methodology we proactively use at the very start of the product realization process. Each component we design and specify is optimised for ease of assembly and manufacturing. Both of these aspects have a high impact on the final product’s quality and cost. Factors such as raw materials, manufacturing processes, volume, machinery, tooling, precision, number of parts and their complexity, labour and skills, and automation potential are all considered.
We keep the design of components simple, modular and standardized where possible, select suitable tolerances and incorporate mistake-proofing techniques (poka-yoke) to improve the accuracy rates of assembly and manufacturing operations. Using DfMA principles and reducing complexity makes for a seamless and successful transition to manufacturing. We have an established supply chain network which comprises of some of the best suppliers around the world and access to the latest specialist materials, technological advancements and expertise. Our ISO 13485 quality management system supports Good Manufacturing Practises (GMP) ensuring that products are consistently produced and controlled according to specified quality standards.


Regulatory Compliance
In our field of operation regulatory compliance and quality assurance is integral to everything we do. BG Research is an ISO 13485 certified company (certified since 2020). Our accreditation is not simply treated as an accolade, instead it is installed within the culture of the company and our employees.
Our regulatory compliance and QA team ensures our processes, documentation and products meet the latest applicable regulatory requirements. This includes risk management, submission of design dossiers, clinical validation and post-market surveillance covering the entire life cycle of medical devices. Our goal is to get products to market that meet the stringent requirements of regulating bodies and also those of our customers.
Project Management
Effective project management has been a key foundation to our success as a company. We are able to seamlessly co-ordinate projects ensuring defined milestones and deliverables are met in accordance with planned timelines.
BG Research has completed an extensive number of projects with partners from around the world (e.g. Public Health England, The Pirbright Institute, USAMRIID, INMI and Marseille University) which in turn have generated an extensive portfolio of game-changing molecular and engineering technology based solutions. This has also allowed us to build a powerful global partner network meaning capabilities, resources and specialist facilities can be collectively leveraged to advance scientific discovery and significantly improve global health outcomes.




